& Pharmaceutical
External and industry forces reshaping life sciences enterprises
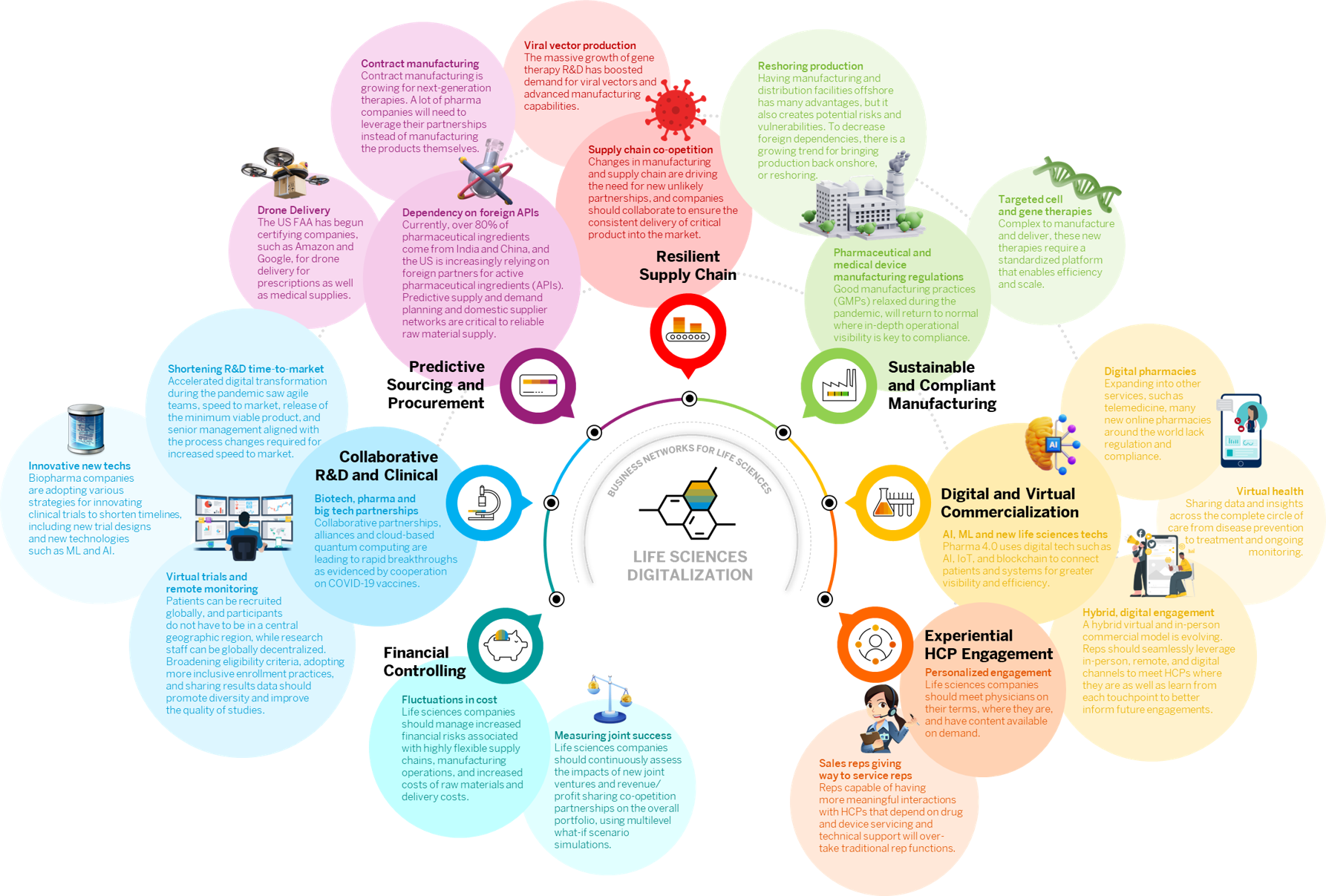
Life sciences companies will continue to evolve business models in a more patient lifecycle service-based context, such as personalized genome therapy treatments. The nature of biopharmaceutical R&D and medical device design and engineering processes will increasingly become patient-centric and require collaborative networks to accelerate time to market. Traditional back-office functions such as finance, IT, HR, data management, and commercialization must continue to matured into a single global service organization that supports HCP value chain relationships. Manufacturing, supply, and logistics networks will be smart, flexible and agile to support co-opetition joint ventures. Successful business model innovation, process optimization, and workforce productivity are intrinsically linked as the new norms. Embracing new technologies and implementing the right business initiatives will form the foundation for innovative business capability expansion.
Industry Insight
Empowered patients are accelerating personalization
Patients are taking control of their health and are demanding therapies that provide promised outcomes. Personalized medicines are emerging at faster rates, with higher price points and improved patient results. Being able to provide outcome-based patient engagements and connect with patients directly becomes paramount. Life sciences companies will increasingly identify customer segments and service them directly for more holistic approaches that improve quality of life. Radical transformation of the supply chain and manufacturing processes will enable greater agility in supporting targeted therapy treatments, personalized patient approaches, and smaller product segments, as well as provide real time views of patients and their interactions. This means the patient will be served seamlessly by hospitals and clinics for therapy, even when there is some disruption to the ways distribution models work through wholesale and retail chains.

Industry Insight

Co-opetition in the global life sciences ecosystem
As public health issues continue to arise with their associated impact on society, cooperation and competition come together to produce collaborative solutions to our world’s most pressing challenges. The COVID pandemic proved how efficiencies through collaboration between competitive enterprises from across the life sciences network could be employed to speed time-to-market at reduced costs.
Life sciences companies will continue to collaborate more closely with manufacturers and suppliers to help ensure quality standards meet regulatory compliance on ingredients, packaging, and finished products.
Collaborating on product design across the extended network of research institutes, hospitals, and innovative startups will further enrich products to meet patient needs when needed most.
Industry Insight

Enabling the digital supply chain and smart interconnected manufacturing
Supply chains and manufacturing networks must be able to seamlessly execute varying manufacturing strategies and respond directly to demand signals and customer orders. This requires increased automation and intelligence on the shop floor, including continuous process verification; artificial intelligence to check the status of chemical and biological reactions; smart warehouse functions; and error reduction through automated processes such as e-labeling.
Manufacturing compliance will return to Good Manufacturing Practices (GMPs) that will impact the global supply chain from availability of active pharmaceutical ingredients to overseas production and contract manufacturing and reshoring.
Industry Insight

Innovating commercialization with digital, hybrid engagement
Society has shifted from onsite to virtual seemly overnight. And the life sciences commercial model is no exception. Digital enablement has become a necessity, with shifts to completely virtual models. Digital transformation of the commercial model will lead the shift from onsite to virtual engagement through new creative approaches to serve.
Face-to-face promotions will increasingly be designed to augment virtual engagement, requiring more meaningful and readily accessible practical content and service. HCP relationships will be fostered by content that resonates with real-time needs. In the new norm, life sciences companies will meet physicians on their terms, where they are, and have content available on demand to support HCP and patient educational needs alike.
Industry Insight

Speeding R&D time-to-market lifecycles
“Necessity is the mother of invention.” The pandemic demonstrated R&D acceleration was indeed possible through near instant digital transformation that spun-up agile teams, released rapid minimum viable products, and drove collaboration inside large corporations let alone between industry stalwarts.
The rapid development of new vaccines demonstrated that streamlining was indeed possible. The pandemic exposed long-standing inefficiencies within biopharma R&D operating models, and now there is greater commitment and confidence to accelerate new drug development timelines. Biopharma companies are adopting digital strategies for innovating clinical trials to shorten timelines, including the use of new cloud-based technologies, such as ML and AI.
SAVANTIS LIFE SCIENCES CUSTOMER PROFILE


Huahai Pharmaceutical converts operations to Savantis SAP S/4HANA on AWS Cloud
Founded in 1989, Huahai Pharmaceutical Co., Ltd. is headquartered in Zhejiang, China and focuses in the chemical and biological drug, pharmaceutical packaging and trading treatment market segment. Huahai employs over 6,800 employees worldwide. Throughout our 5-year partnership, we have engaged with Huahai Pharmaceutical to lead numerous strategic SAP initiatives. This began in 2016 with the original SAP implementation to support their entire back-office processes. Since then, Savantis has successfully managed and upgraded the solution—to meet rigorous compliance standards of the worldwide life sciences industry protocols.
ENABLING THE NEW DIGITAL ECONOMY FOR THE LIFE SCIENCES, BIOTECH AND PHARMACEUTICAL INDUSTRY USING SAP® CLOUD-BASED SOLUTIONS

For twenty-five years Savantis has been at the forefront of SAP Life Sciences, implementing and supporting SAP global platforms for enterprises around the world. Savantis was the first system integrator to convert to an S/4HANA system in North America. Our depth and breadth of SAP Life Sciences experience covers the entire S/4HANA and CX suite including, ecommerce, digital marketing, CRM, sales management, sourcing and procurement, research and development, logistics and transportation management, inventory management, manufacturing, and finance.
Is your company an intelligent enterprise?
Savantis is a full-service SAP global systems integrator focused on one common goal—success! If you have had your fill with the larger tier one integrators but still need a holistic global services provider, Savantis is your answer. We have the depth and breadth of SAP experience to deliver and support global enterprise solutions. Our delivery leadership has the tier one experience at an affordable price point unmatched in the SAP ecosystem. Come experience the difference.